sk life science xcopri
Cenobamate is an anti-seizure medication ASM discovered and developed by SK Biopharmaceuticals and SK life science. Xcopri cenobamate is a member of the carbamate anticonvulsants drug class and is commonly used for Seizures.
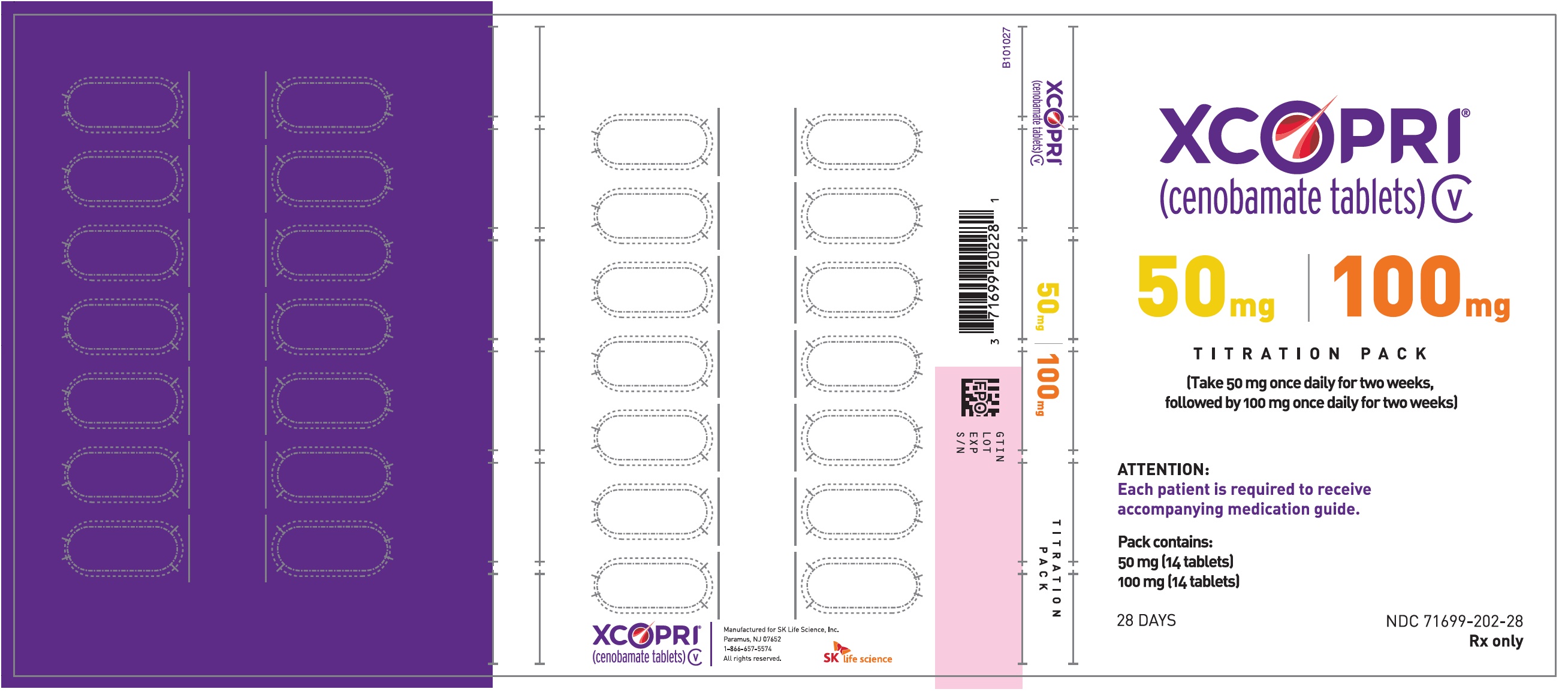
Yes Xcopri is a schedule 5 controlled substance C-V first approved in 2019.
. Learn about the medicines we have today as well as those that are currently in development. SK life science is committed to the development of next generation treatment for CNS disorders. He suffered from 24 seizures a year and this continued until he joined a clinical trial of SKs now approved epilepsy drug Xcopri which got him where is he is todayzero seizures.
Program managed by ConnectiveRx on behalf of SK life science. SK life science reserves the right to rescind revoke or amend this program at any time for any reason without notice. 3 2021 PRNewswire -- SK Life Science Inc a subsidiary of SK Biopharmaceuticals Co Ltd an innovative global pharmaceutical company focused on.
Additionally SK life science will sponsor a product theatre session TV News Anchor Sarah Carlsons Treatment Journey with XCOPRI where physician Michael C. PARAMUS NJ Dec. We explore the complex mysteries of the brain to find.
Still work to be done. It is an oral tablet used for the treatment of partial-onset seizures in adult patients with. As long as there are unmet needs in CNS we keep working.
It is for informational purposes only and not intended as medical advice. One of SK Life Sciences trials for instance showed a 56 reduction from baseline in median seizure frequency compared to a 22 reduction among a placebo group over 12. The cost for Xcopri oral tablet 250 mg daily-dose is around.
About XCOPRI cenobamate tablets CV Cenobamate is an anti-seizure medication ASM discovered and developed by SK Biopharmaceuticals and SK life science. Because theresstill work to be done. SK Life Science Inc a subsidiary of SK Biopharmaceuticals Co Ltd an innovative global pharmaceutical company focused on developing treatments for central.
The content on this site provides non-promotional scientific data on SK life science products. Its boots on the ground or rather in the office for SK Life Sciences latest sales push aimed at getting its target ie neurologists to pay attention. About XCOPRI cenobamate tablets CV.
Its boots on the ground or. About XCOPRI cenobamate tablets CV Cenobamate is an anti-seizure medication ASM discovered and developed by SK Biopharmaceuticals and SK life science.

Cenobamate Headed To Market For Partial Onset Seizures After Dea Scheduling

Sk Life Sci Kicks Xcopri Sales Into High Gear With New Campaign

Ndc 71699 150 Oral Tablet Film Coated Xcopri Drug Codes Packaging Active Ingredients

Sk Life Science Receives Schedule V Designation From Dea For Xcopri Cenobamate Tablets
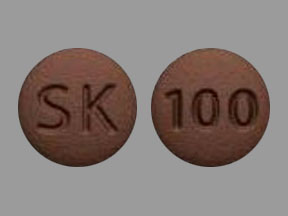
Sk 100 Pill Brown Round Pill Identifier Drugs Com

Medical Information Sk Life Science Medaffairs
Fda Approves Cenobamate For Partial Onset Seizures

Sk Life Science Receives Schedule V Designation From Dea For Xcopri Cenobamate Tablets Pharmiweb Com

Gary Ball Vp Sales Marketing Sk Life Science Inc Linkedin

Xcopri Package Insert Prescribing Information Drugs Com
South Korea S Sk Biopharmaceuticals Plans To Raise 850m In Ipo Financial Times

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Practical Guidance For The Management Of Adults Receiving Adjunctive Cenobamate For The Treatment Of Focal Epilepsy Expert Opinion Epilepsy Behavior

Cenobamate Treatment Results In Sustained And Meaningful And Profound Seizure Reduction Across Multiple Partial Onset Focal Seizure Subtypes Practical Neurology

Cenobamate A New Treatment Option For Partial Onset Focal Seizures Cure Epilepsy

Xcopri Rush Neurology Virtual Conference

2021 Pharma Choice Professional Website Bronze Winner Cdmp And Sk Life Science Inc Pm360

Big Korean Corporate Names Enlarge Footprints In Bio Market Pulse By Maeil Business News Korea